Crohn’s disease (CD) is a non-communicable, global disease with an accelerating incidence (up to 23 per 100,000 persons/year) and annual health costs in excess of 5 billion euro in Europe. Despite advances in new immune modulators and biological treatment, up to 30% of patients become non-responders, highlighting the pressing need for novel, advanced therapies.
GENEGUT will transform the treatment of ileal CD by developing a first-in-class oral RNA-based therapy, tackling inflammation locally in the intestinal tissue, while avoiding systemic side effects. The success of COVID-19 mRNA vaccines exemplified the potential of RNA-based therapies. Now, the challenge is to extend this revolutionary therapeutic modality to non-communicable diseases where RNA delivery has not been achieved, such as ileal CD.
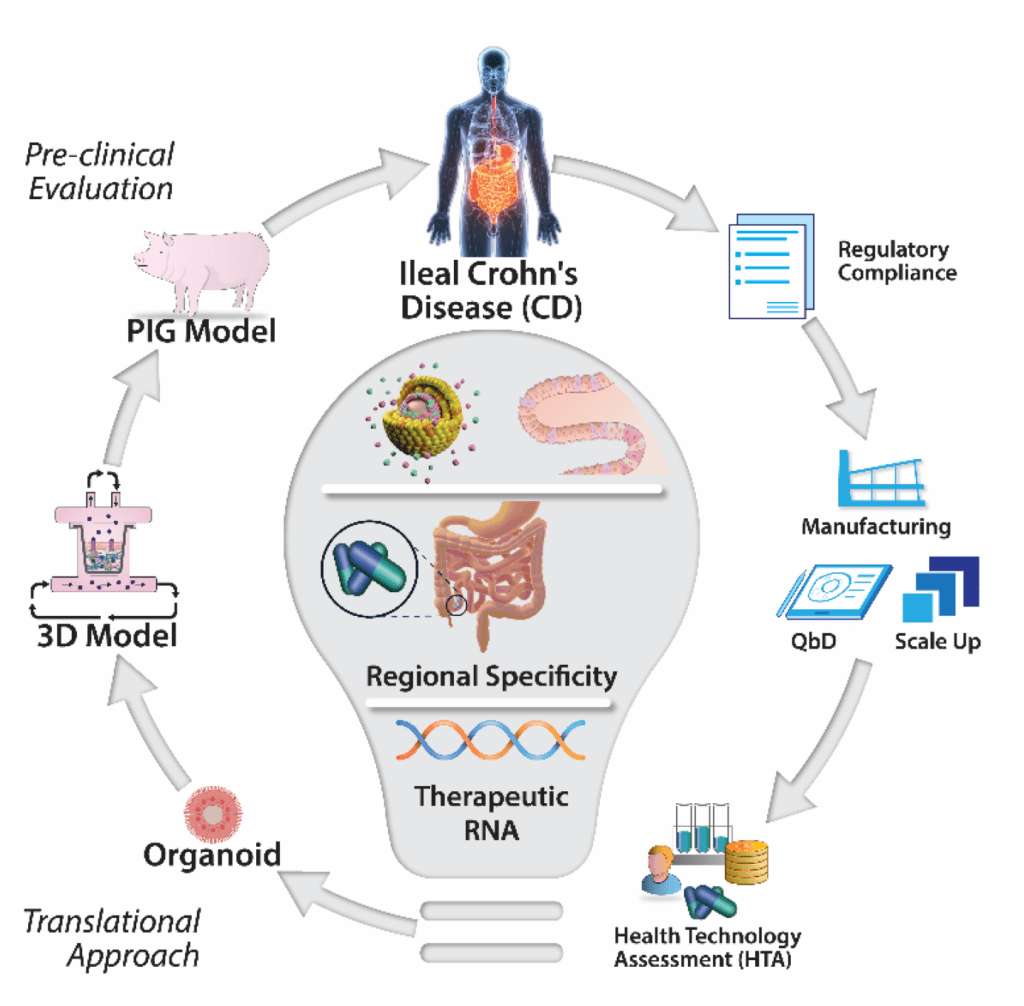
Delivery of RNA will be enabled by a combination approach where novel biomaterials will complex the RNA within nanoparticles (NPs) designed to overcome the barriers in the gastrointestinal tract. Our industry partner’s (Lonza) novel capsule technology will ensure site-specific release of the encapsulated NPs at the diseased ileum. The RNA-nanotherapeutics (siRNA and mRNA) will modulate gene expression locally in the ileum by targeting the clinically validated (JAK/STAT) pathway and two exploratory targets. Proof-of-efficacy will be demonstrated in vivo and also in a 3D organoid and multi-cellular model of ileal CD using primary human cells. GENEGUT brings together 9 partners from 8 European countries covering the lifecycle from bench to bedside, creating a network of renowned researchers, expert clinical scientists, SMEs and larger pharma companies and a patient organisation (EFCCA). GENEGUT will result in an oral RNA therapeutic ready for early clinical trial including coherent plans for clinical trials and regulatory submission thus enabling rapid availability for patients.
GENEGUT's approach
Three molecular pathways for the RNA-based gene modulation have been identified including JAK/STAT and two exploratory pathways including a peptide hormone regulating inflammation and barrier function and a glycoprotein regulating cellular proliferation and fibrosis. Libraries of novel biocompatible materials, will be synthesised to formulate NPs that encapsulate relevant RNA cargos. Targeted oral delivery of RNA therapies to the ileal region will be achieved by encapsulating NPs within our industry partner’s (Lonza) emergent capsule platform technology. Pivotal proof-of-efficacy will be demonstrated in an in vivo pig model of chronic intestinal inflammation and also a 3D organoid and multi-cellular model of ileal CD using primary human cells. The novel experimental data generated will then be integrated into a suite of tools to promote faster access for patients to this breakthrough therapy, including pharmacokinetic models to predict localised tissue concentration in diseased patients and a Health Technology Assessment to guide pharmacoeconomic outcomes. The robust data provided from the efficacy trials in pre-clinical and disease-relevant cell models will inform the design of subsequent clinical trials and related protocols.

